Behind the Breakthrough: Patent FAQ
1. What was just announced?
Dr. Shai Berlin, an NMDA receptor expert part of a collaborative network of laboratories supported by the 2BCured Foundation, made an important scientific breakthrough relating to GRIN2B-Related Neurodevelopmental Disorder resulting in the issuance of a patent.
2. How does the therapy work?
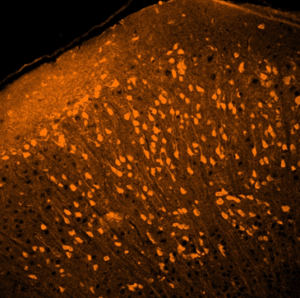
The therapy uses a safe, modified virus known as AAV (adeno-associated virus) to deliver a healthy copy of the GRIN2B gene directly to the brain. Think of AAVs as tiny delivery trucks that carry the healthy gene into brain cells. This allows the brain to make the protein it’s missing due to the GRIN2B mutation. The goal is to help brain cells (neurons) function normally by restoring proper activity of the NMDA receptor, which is affected in GRIN2B-Related Disorder.
This image from Dr. Berlin’s lab shows neurons expressing the healthy GRIN2B gene (marked by a yellow color), which they received through AAV therapy.
3. Why is this considered a breakthrough?
Until now, the GRIN2B gene was thought to be too large to fit inside an AAV, which is commonly used for gene therapies. This is the first time scientists have successfully packaged the entire GRIN2B gene into an AAV and safely delivered the full gene into the brain of lab models—something that has never been done before.
4. Has the therapy been tested?
Yes, early preclinical tests (in animal models and lab-grown brain cells) have shown that the gene reaches neurons and becomes active. Importantly, these early studies also showed no signs of toxicity or harm to neurons.
5. Does this mean a treatment is available now?
No, not yet. This is a full-scale proof of concept (PoC), a major step forward that helps accelerate the path toward a cure. Reaching PoC often takes years, especially for rare or novel genes like GRIN2B. While it’s a key milestone, FDA approval and clinical use are still several years away.
6. When could this be available for individuals with GRIN2B?
It’s too early to give a timeline. The research team is working to move the project forward, but this process can take several years. Support from pharmaceutical partners, advocacy groups, and families will be important in accelerating the next phases.
7. How does gene addition using AAV work, and why is it considered a promising approach for treating genetic diseases like GRIN2B?
Gene addition using AAV (adeno-associated virus) works by delivering a healthy copy of a gene to cells where the original gene is missing or not functioning correctly. Rather than trying to repair the faulty gene, this approach bypasses it entirely by introducing a new, working version.
Key Benefits:
- It targets the underlying genetic cause of the disease, not just the symptoms.
- A single treatment can lead to long-lasting expression of the therapeutic gene.
- This method is already clinically validated and approved for several neurological and systemic genetic disorders.
As more success stories emerge in the field, there’s growing hope that GRIN2B will join the list of conditions treatable with approved genetic therapies.
8. How can families help?
Support from the community is essential. Parents are encouraged to spread awareness of our work, stay informed by signing up for our 2BCured newsletter, participate in registries and natural history studies, and encourage collaboration with pharmaceutical companies.
Parents who are interested in contributing their expertise—whether in communications, fundraising, media, legal, or medical fields—are encouraged to reach out by emailing info@2bcured.org.
9. What’s next for the research team?
The next steps include:
- Expanding safety testing
- Optimizing the dose of the therapy
- Scaling up production to prepare for future studies
The team is also exploring partnerships to help move toward clinical readiness.
We’ll continue to share updates as progress unfolds. Thank you for being part of this journey toward meaningful treatment for GRIN2B Disorder.